iClinical: Data platform for virtual clinical trials
As part of i3 Consult’s partnership with iClinical, we are pleased to announce the launch of iClinical’s Data Platform for Virtual Clinical Trials mobile app. This technology is destined to leverage clinical trial management and outcomes through data collection at the source of the trial app with secure FDA 21 CFR Part 11 compliance and real time analytics and trial automation. This application can reduce 20-30% clinical trial costs and accelerate the timeline to regulatory approval and market launch by 6 to 8 months. Therefore we look forward to introducing this smart and unique technology to our clinical trial business space.
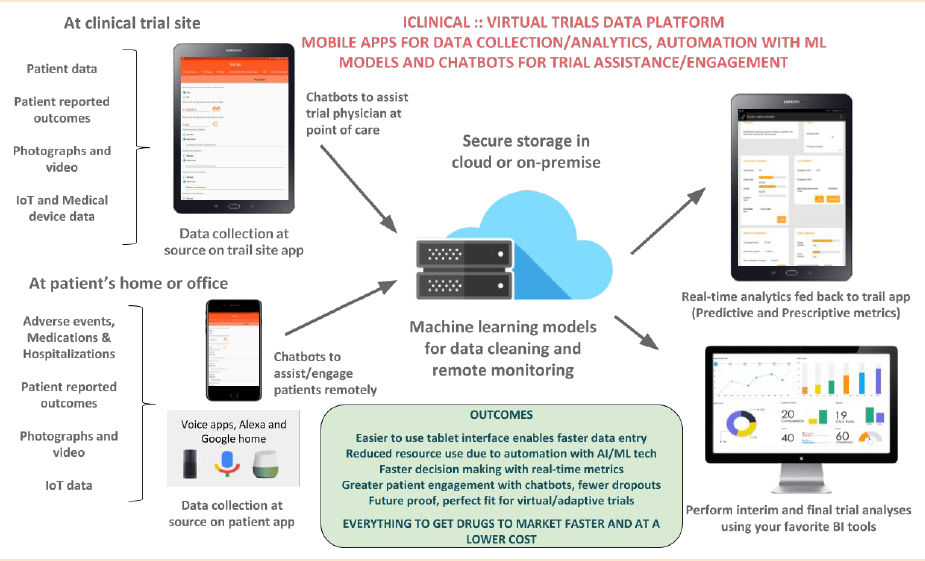 Data collection at source on trial apps
Data collection at source on trial apps
➔ Data collection happens in front of the subject (eSource). Any patient reported outcomes, video, audio and medical devices data is collected at source.
➔ The AI based trial assistant (chatbot) enables real-time retrieval of protocol related information, it enables principal investigators to see more patients in lesser time.
➔ With reduced data cleaning and remote monitoring, we can drastically reduce study costs.
Secure and FDA 21 CFR Part 11 compliant
➔ Secure storage is in HIPAA compliant cloud servers. There is complete 21CFR Part 11 compliance, including audit trail of all the entered data, investigator signatures and secure access to the study tablets.
Real time trial analytics and trial automation
➔ Predictive analytics provides Pharma’s with the trajectory of the trial in real-time. This includes the endpoints, site status and study outcomes.
➔ Machine learning models are used to clean the trial data at source, they are also used to generate holistic patient profiles and optimize remote risk based monitoring.
Outcomes
➔ With reduced data cleaning and remote monitoring, we can drastically reduce study costs. For a typical phase 3 clinical trial, we can save 20-30% of trial costs and get the drug to market sooner by 6-8 months.
➔ The platform is future proof and is perfectly suited for running remote and adaptive trials.
Products like this stand testimony to the success of our partnership with iClinical. This indeed will be a game changer for delivery of projects with our clients and i3 Consult look forward to working with iClinical in implementing this technology. For further details please and to request a private demonstration kindly contact Sridhar Byrappa PhD, CEO of iClinical as in the contact details provided:
Sridhar Byrappa PhD
CEO, iClinical Inc
Ph: 415-996-6860
Email: sridhar@iclinical.co
Website: http://iclinical.co/
LinkedIn:https://www.linkedin.com/in/sridharbyrappa/
Best regards,
Dr. Wallace Macindoe,
CEO & Founder of i3 Consult.
i3 Consult- Integrated Intelligence for Healthcare Industries.