Case Study: Structure, Function, Roles and Responsibilities for a Small Cap Medical Device Company
The Challenges: For a fast-growing medical device company having a global presence, there are challenges both internal and external to the organization. Being responsive to the regulatory, quality and safety issues, across multiple global markets can be overwhelming to the executives of a small cap medical device company. Internally medical device company executives are held accountable to reach certain milestones set by Director of the Board (e.g. study completion, licensing approval in key markets, publication of proof of concept data, sales turnover targets, to name just a few) and/or are tasked to deal with company-wide issues which may be either technical, regulatory, or business related. Outsourcing to a consultant with vertical knowledge in a specific functional area is a standard approach in systematically trying to meet the milestones set or deal with the issues faced. Often however, faced with the challenges of trying to integrate this consultancy-based decision making, using the so-called compartmentalized approach, results in milestone shortfalls, critical success issues being only partly resolved or remaining unresolved, protracted timelines, and overspending of the corporate budget.
An Optimal Strategy: A small cap medical device company needs to have its organizational structure, functional roles and a comprehensive set of SOPs in place developed from their regulatory strategy and quality management system. Their regulatory strategy and quality management system can be developed from a cross-functional structure of their organization which would co-ordinate the functional area talent focused on sharing the ‘Bigger Picture’ of long term corporate goals. This approach of tying in structure, functional roles and SOPs with regulatory strategy and quality management systems has repeatedly proven effective in putting medical device companies on the ‘fast track’ for cost and risk reduction as well as championing the competitive advantage.
Action to be Taken: It is proposed to the small cap medical device company, in all three areas of structure, function and responsibilities of its organization, development and deployment of an appropriate regulatory strategy, the most efficient and compliant quality management system, and then to aggressively execute these actions needed to demonstrate product efficacy and safety under a corrective feedback system. Specific structures, functions and responsibilities for middle to executive management levels are needed to ensure that there is a seamless work flow in and around the components of the organizational structure shown (Figure 1).
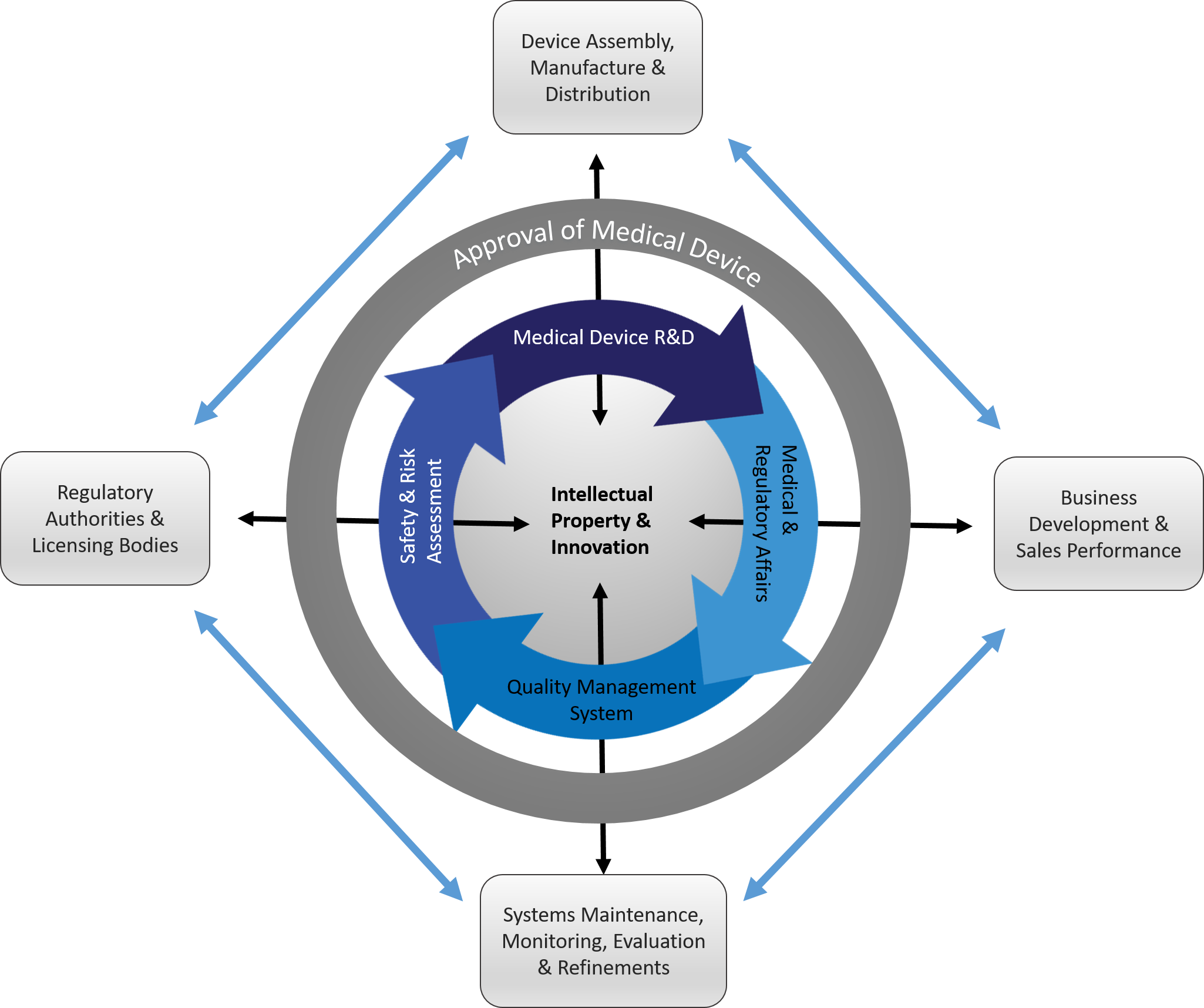
Figure 1: Organizational Structure of a Medical Device Company